Abstract
In breast conserving surgery (BCS), specimen mammography is one of the most widely used intraoperative methods of assessing margin status. We performed a meta-analysis to evaluate the diagnostic accuracy of specimen mammography. Literature databases including PubMed, Cochrane Library, Web of Science, and EMBASE were searched prior to Jun 2022. A total of 1967 patients were included from 20 studies. A pooled analysis, heterogeneity testing, threshold effect testing, publication bias analysis, and subgroup analyses were performed from extracted data. The pooled weighted values were a sensitivity of 0.55 (95% confidence interval [CI], 0.47–0.63), a specificity of 0.85 (95% CI, 0.78–0.90), a diagnostic odds ratio of 7 (95% CI, 4–12), and a pooled positive likelihood ratio of 3.7 (95% CI 2.6–5.5). The area under the receiver operator characteristic curve was 0.75 (95% CI 0.71–0.78). In the subgroup analysis, the pooled specificity in the positive margin defined as tumor at margin subgroup was lower than the other positive margin definition subgroup (0.82 [95% CI: 0.71, 0.92] vs. 0.87 [95% CI: 0.80, 0.94], p = 0.01). Our findings indicated that specimen mammography was an accurate intraoperative imaging technique for margin assessment in BCS.
Introduction
Breast cancer is one of the most common cancers worldwide. An important surgical modality for women with early-stage breast cancer is breast-conserving surgery (BCS). BCS entails removal of the cancer and sparing of the rest of the breast, balancing oncologic resection, and providing an ideal cosmesis. In recent years, more patients are diagnosed with smaller invasive tumors and are therefore likely to receive BCS. The use of neoadjuvant chemotherapy results in tumor downstaging, which leads to more patients undergoing BCS. Achieving a negative margin is critical in BCS, as a positive margin is one of the most significant risk factors for local recurrence. A positive margin occurs in 20–40% of all patients and 15–30% patients undergo reoperation to improve local control1,2,3,4. Reoperation is associated with a higher surgical risk, poorer cosmetic outcome, and increased psychological and economic burden5.
Histopathology is the gold standard for margin assessment. However, the analysis is performed post-operatively. Intraoperative margin assessment methods have been developed to identify positive margins and to enable immediate re-excision, thereby decreasing the reoperation rate. These methods mainly consist of two types, pathological methods, and imaging methods. Intraoperative pathological assessment methods, such as frozen section and imprint cytology, are time-consuming6. Intraoperative imaging methods have emerged aiming at real-time intraoperative management7.
Specimen mammography is usually used to confirm the excision of the targeted lesion. It is also an intraoperative resection margin assessment method performed during BCS. Two kinds of systems mainly exist in performing specimen mammography, that is, conventional specimen radiography (CSR) and intraoperative digital specimen mammography (IDSM). CSR is a widely used system in which the specimen is transported from the operating room to the radiology department for specimen radiography. CSR also requires compression of the surgical specimen between mammography plates which may impair its diagnostic accuracy8. Another system, IDSM, is developed by using a small automatic device placed near the operation room to take images, which helps to save operation time. Specimen compression is not needed in IDSM. Multiple views can be obtained and specimens can be placed with the proper orientations, as IDSM is often performed by the operating surgeon. IDSM may have some potential advantages, such as less anesthesia, less cost, predictable operating room schedule, reduced re-excision rate, and less tissue excised9,10,11.
Specimen mammography is widely used to assess margin status intraoperatively. However, its diagnostic accuracy remains controversial. Some studies have shown that specimen mammography was reliable in identifying clear margins and reduced the rate of reintervention12, while others have indicated it to be not accurate enough13,14. We performed this meta-analysis aiming at evaluating pooled diagnostic accuracy for specimen mammography in assessing margin status during BCS.
Methods
Our study was conducted following guidelines of the “Preferred Reporting Items for Systematic Reviews and Meta-Analyses” (PRISMA)15. We searched relevant studies in Pubmed, Cochrane Library, Web of Science, and EMBASE prior to Jun 2022. The search strategy comprised both keywords and MeSH terms for “breast cancer” (MeSH term of Breast Neoplasms, breast cancer*, breast tumour*, breast tumor*, breast neoplasm*, Breast carcinoma*, mammary cancer*, mammary tumour*, mammary tumor*, Mammary Neoplasm*, mammary carcinoma*), “specimen mammography” ((mammograph* OR radiograph* OR X-ray) AND specimen*) and “margin*.” All types of articles were included. Extensive crosschecking of the references in retrieved articles was performed. Two investigators (Chen Lin and Kaiyue Wang) screened the titles and abstracts independently. All relevant citations were selected for further analysis. When a disagreement between two investigators regarding article inclusion surfaces, they discussed these articles with a senior author (Tao Pan). All three authors are surgeons.
Studies were included when they met the following criteria: (1) patients were diagnosed with breast cancer (invasive or in situ) and received BCS; (2) specimen mammography was performed intraoperatively to assess the margin status; (3) studies provided available data of true positive (TP), true negative (TN), false positive (FP), and false negative (FN). Studies not written in English were excluded. Opinions, case studies, reviews, conference abstracts, and meta-analyses were also excluded. Full-texts of the eligible studies were reviewed. The following information was extracted: (1) the first author; (2) year of publication; (3) number of patients; (4) number of lesions; (5)study design; (6) mean age of patients; (7) histopathological definition of positive margin distances; (8) positive margin rates; (9) mammography system used in the article; (10) diagnostic accuracy raw data- TP, TN, FP, and FN; and (11) percentages of sensitivity, specificity, positive predictive value, and negative predictive value.
Quality Assessment of Diagnosis Accuracy Study (QUADAS-2) form was used to assess methodological quality of the eligible studies16. QUADAS-2 checklist was independently completed by the two investigators (Hailang Chen and Yuhua Xu).
The relationship between sensitivity and specificity was assessed using a hierarchical summary receiver operating characteristic (SROC) model. The area under the curve (AUC) of the SROC was calculated to measure the diagnostic performance of specimen mammography. An AUC between 0.9 and 1.0 was considered a very good degree of diagnostic accuracy, between 0.7 and 0.9 represents a moderate degree and an AUC close to 0.5 AUC implies a poor degree17. The Youden index (*Q), which is used in conjunction with SROC analysis and recognized as a preferred statistic to reflect the diagnostic value, was also assessed. A *Q index of 1 indicates a perfect test result. Heterogeneity was assessed using I2. 25–49% was considered low level, 50–74% was moderate, and > 75% was high. A random effects model was used when I2 > 50%, and a fixed effects model was chosen when I2 < 50%18. The threshold effect was one of the important sources of heterogeneity in diagnostic accuracy tests. The Spearman correlation coefficients can determine the existence of the threshold effect if the p-value ≤ 0.05. Publication bias was analyzed using Deeks’ funnel plot. The absence of a non-zero slope coefficient (P < 0.10) indicates that a publication bias exists among the included studies19. A subgroup analysis was performed. Statistical software package Revman 5.3 and Stata version 16.0 (StataCorp, College Station, Texas) were used.
Results
Search results and study selection
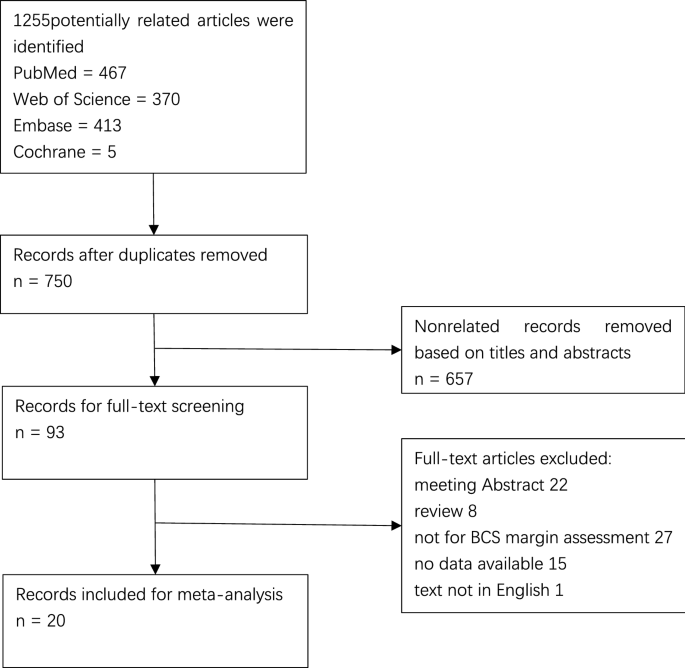
The systematic search and manual crosschecking of references yielded 1255 articles from PubMed, Web of Science, Embase, and Cochrane initially. After duplicates were removed, 750 unique records remained. After excluding the obviously unrelated articles by reading the titles and abstracts, 93 remained as potential articles. An additional of 73 articles were excluded after a careful full-text review. The reasons for exclusion were as follows: (1) TP, TN, FP, and FN data were missing; (2) the paper did not focus on specimen mammography for margin assessment; (3) the paper was published in abstract format only; (4) the paper was a literature review; and /or (5) the paper was not written in English. Eventually, 20 studies were enrolled in the analysis12,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38.
Study description
Our meta-analysis included 20 studies which consisted of a total of 1967 patients and 1988 lesions (Fig. 1) . The sample size ranged from 34 to 267 patients and 34 to 280 lesions. The mean or median age was available in 12 studies with a range between 50 and 60 years old. Sixteen studies mentioned the system used to take specimen mammograph. Eleven of the studies used the CSR system, three used the IDSM system and two used both systems for margin status assessment in BCS. Four studies did not provide information about the applied system. In seven studies, radiologists were blinded to the pathological results. The basic information and principal characteristics of the included studies including the number of patients and lesions, method of studies, mean age of patients, negative margin distance, positive margin rates, and mammography system are detailed in Table 1. Documented or calculable percentages of sensitivity, specificity, positive predictive value, and negative predictive value for each study are listed in Table 2. According to QUADAS-2 items, the quality assessment of 20 studies was moderate. The results of the distribution are shown in Supplementary Fig. S1.
Meta-analysis
The statistical results confirmed a moderate and high heterogeneity of specimen radiography for sensitivity (I2 = 65.48%) and specificity (I2 = 93.05%), respectively. A random effects coefficient binary regression model was used. The pooled estimate of 20 studies demonstrated a sensitivity of 0.55 (95% CI, 0.47–0.63), a specificity of 0.85 (95% CI, 0.78–0.90) (see Supplementary Fig. S2), and a diagnostic odds ratio of 7 (95% CI, 4–12). The pooled positive likelihood ratio was 3.7 (95% CI 2.6–5.5) and the pooled negative likelihood ratio was 0.52 (95% CI 0.44–0.62). The AUC value, which represented the overall diagnostic accuracy of specimen radiography, was 0.75 (95% CI 0.71–0.78). The *Q index was 0.6929 ± 0.0380. The SROC graph with the 95% confidence region and 95% prediction region is shown in Supplementary Fig. S3.
The Spearman correlation coefficients’ p values (0.779, p > 0.05) disclosed the absence of a threshold effect. Deeks’ funnel plot asymmetry test was performed and no significant publication bias (p = 0.26) existed in the enrolled studies (see Supplementary Fig. S4).
The results of the subgroup analysis were presented in Table 3 and Supplementary Fig. S5. BCS had been performed for decades. In the early period, there was no consensus on what constitutes an optimal negative margin width39. The various definitions of negative margin included "no tumor cells on the ink", no tumor cells seen at < 1 mm, < 2 mm, and larger distances (> 5 mm)40. The negative margin distances varied among the included studies. The studies were divided into two subgroups. All studies with a negative margin distance of 0 mm were included in the no tumor at margin subgroup. The remaining studies were included in the other negative margin definition subgroup. The subgroups had comparable pooled sensitivity (0,58 [95% CI: 0.45, 0.71] vs. 0.53 [95% CI: 0.43, 0.64], p = 0.95), but the pooled specificity was significantly lower in the no tumor at margin subgroup (0.82 [95% CI: 0.71, 0.92] vs. 0.87 [95% CI: 0.80, 0.94], p = 0.01).
Discussion
Histopathological results of the margin status occupy a particularly important place in BCS. The application of an accurate intraoperative diagnostic method can reduce the reoperation rate. In recent years, numerous studies have compared the ability of techniques in detecting positive margins to the final histopathological results. Assessing the diagnostic accuracy of intraoperative methods is necessary.
Intraoperative pathological and imaging methods are the two main methods for intraoperative margin assessment in BCS41. Intraoperative pathological methods mainly include frozen section, touch smear, and imprint cytology, which all have high sensitivity, specificity, and accuracy42. Despite the high level of accuracy, intraoperative pathological techniques are infrequently employed and depend heavily on the pathologists’ experience. Pathological methods are also time-consuming. They often add an average of 20–30 additional min to the operation time6. Intraoperative imaging methods can quickly assess margin status and reduce operation time. Specimen mammography is one of the most widely used intraoperative imaging methods. Various studies are available in the literature to evaluate the value of specimen mammography in margin status assessment. The findings of our meta-analysis indicated that the diagnostic accuracy of specimen mammography was promising, with an AUC of 0.75, despite some limitations.
There was moderate and high heterogeneity for sensitivity and specificity, respectively, in our meta-analysis. The heterogeneity among studies may be attributed to factors, such as study design, type of specimen mammography system, and different definitions of negative margin. Two types of systems mainly exist which are used to take specimen mammography, namely, CSR and IDSM. A two-dimensional CSR is a technique used routinely in many cancer institutions. The device used for performing CSR is usually is the same one used for patients’ mammography. The device is often located in a radiology unit. Surgical specimen needs to be transported from the operating room to the radiology department, which makes it labor-intensive and time-consuming. The disadvantages of CSR also include low specificity, as additional excision of the tissue is often recommended unnecessarily43. Specimens examined with CSR are often compressed with a plate, to simulate the conditions of the mammographic examinations22. Kyle Ota believed that the high false positive rate may be caused by “pancake phenomenon43”, where CSR compresses the surgical specimen. This manipulation refers to a reduction in the mean volume and height of the breast specimens which may increase FP margins8. The other widely used system is IDSM. It is a portable, dedicated, self-contained digital imaging system, often placed near the operation room. Without transporting the specimen to the radiology department, IDSM shortens the specimen transport time. IDSM results can be read by the surgeon near the operation room. Real-time review shortens the time required for radiologist review. When the radiologist is not immediately available, the surgeon can review images and make decisions. However, it acquires additional skills in learning image interpretation. The IDSM system significantly reduced the operative time for BCS compared to CSR26, which in turn decreased anesthesia time and operation room cost. Aside from the reduced time, some studies have found a reduction in the reoperation rates after the introduction of IDSM12,22. However, in our study, no significant difference was observed between subgroups of CSR and IDSM. Further assessment requires large-scale and well-designed clinical trials.
Different definitions of negative margin may also lead to heterogeneity among studies. Before 2014, controversy exists regarding the optimal margin width in BCS for breast cancer40. The American Society of Clinical Oncology guidelines suggested that tumor margins not touching ink at the specimen edge were acceptable for both invasive cancer and ductal carcinoma in situ44,45. However, a recent study indicated that close margins less than 2 mm were associated with increased local and distant recurrence as well as lower overall survival, compared with wide margins46. Researches are still undergoing to explore an optimum margin width, in order to pursuing lower recurrence rate and better cosmetic outcome. Different margin widths exist among countries and institutions using different guidelines. Among the included studies, a negative margin width differs from no tumor at the margin to no tumor within 5 mm from the margin40. A wider negative margin distance defines those cases with close margins as pathologically positive. “Pancake phenomenon” causes the reduction in mean volume and height of the breast specimens, which also tends to define close margin cases as radiological positive43, leading to better diagnostic accuracy in the wider negative margin subgroup. Our study showed a significant difference in specificity between the two subgroups with higher specificity in the group with wider negative margins.
Besides the factors mentioned above, there might be other factors affecting the diagnostic accuracy of specimen mammography. Goldfeder found that concordance between specimen radiography and histopathology was higher in one-view specimen mammography in comparison to two-view32. Mammographs of specimens were more effective in evaluating the surgical margins of mammographic lesions with microcalcifications than other manifestations47. In some subtypes of breast cancer such as medullary carcinoma, the positive margin signs on specimen mammograph might not represent actual tumor but only a nonneoplastic infiltrate of lymphocytes48.
Other important limitations of intraoperative specimen mammography are: (1) specimen mammography is a two-dimensional imaging method which is inherently flawed in depicting three-dimensional specimens comprehensively41; (2) because of the poor soft tissue contrast, the difficulty in determining the margin status using specimen mammography increases with the breast density49.
Emerging techniques have been developed to improve those limitations. Vacuum intraoperative specimen mammography has been applied to increase the precision of margin detection by decreasing compression, as specimen compression may affect the evaluation of tumor borders50. Three-dimensional imaging techniques, such as digital breast tomosynthesis and micro-computed tomography, have been introduced to solve the dilemma essentially23,51,52. Our meta-analysis data demonstrated a sensitivity and specificity of 0.55 (95% CI, 0.47–0.63) and 0.85 (95% CI, 0.78–0.90), respectively. With a relatively low sensitivity, specimen mammography is less accurate than pathological methods. However, despite those limitations, intraoperative specimen mammography is a widely used and cost-effective procedure for margin assessment. It also helps to reduce the operation time. Specimen mammography can aid in the intraoperative assessment of margin status. Overall, with an AUC value of 0.75, our findings indicated intraoperative specimen mammography to be accurate in assessing margin status during BCS.
Data availability
All data generated or analyzed in this study are included in this published article and its supplementary files.
References
-
Pleijhuis, R. G. et al. Obtaining adequate surgical margins in breast-conserving therapy for patients with early-stage breast cancer: Current modalities and future directions. Ann. Surg. Oncol. 16, 2717–2730. https://doi.org/10.1245/s10434-009-0609-z (2009).
-
Wilke, L. G. et al. Repeat surgery after breast conservation for the treatment of stage 0 to II breast carcinoma: A report from the National Cancer Data Base, 2004–2010. JAMA Surg. 149, 1296–1305. https://doi.org/10.1001/jamasurg.2014.926 (2014).
-
Isaacs, A. J., Gemignani, M. L., Pusic, A. & Sedrakyan, A. Association of breast conservation surgery for cancer with 90-day reoperation rates in New York state. JAMA Surg. 151, 648–655. https://doi.org/10.1001/jamasurg.2015.5535 (2016).
-
Bodilsen, A. et al. The influence of repeat surgery and residual disease on recurrence after breast-conserving surgery: A danish breast cancer cooperative group study. Ann. Surg. Oncol. 22(Suppl 3), S476-485. https://doi.org/10.1245/s10434-015-4707-9 (2015).
-
Houssami, N. & Morrow, M. Margins in breast conservation: A clinician’s perspective and what the literature tells us. J. Surg. Oncol. 110, 2–7. https://doi.org/10.1002/jso.23594 (2014).
-
Butler-Henderson, K., Lee, A. H., Price, R. I. & Waring, K. Intraoperative assessment of margins in breast conserving therapy: A systematic review. Breast 23, 112–119. https://doi.org/10.1016/j.breast.2014.01.002 (2014).
-
Pradipta, A. R. et al. Emerging technologies for real-time intraoperative margin assessment in future breast-conserving surgery. Adv. Sci. 7, 1901519. https://doi.org/10.1002/advs.201901519 (2020).
-
Graham, R. A. et al. The pancake phenomenon contributes to the inaccuracy of margin assessment in patients with breast cancer. Am. J. Surg. 184, 89–93. https://doi.org/10.1016/s0002-9610(02)00902-9 (2002).
-
Kaufman, C. S. et al. Intraoperative digital specimen mammography: Rapid, accurate results expedite surgery. Ann. Surg. Oncol. 14, 1478–1485. https://doi.org/10.1245/s10434-006-9126-5 (2007).
-
Carmichael, A. R., Ninkovic, G. & Boparai, R. The impact of intra-operative specimen radiographs on specimen weights for wide local excision of breast cancer. Breast 13, 325–328. https://doi.org/10.1016/j.breast.2004.01.010 (2004).
-
Kim, S. H. et al. An evaluation of intraoperative digital specimen mammography versus conventional specimen radiography for the excision of nonpalpable breast lesions. Am. J. Surg. 205, 703–710. https://doi.org/10.1016/j.amjsurg.2012.08.010 (2013).
-
Ciccarelli, G. et al. Radiography of the surgical specimen in early stage breast lesions: Diagnostic reliability in the analysis of the resection margins. Radiol. Med. 112, 366–376. https://doi.org/10.1007/s11547-007-0147-3 (2007).
-
Reyna, C. & DeSnyder, S. M. Intraoperative margin assessment in breast cancer management. Surg. Oncol. Clin. N. Am. 27, 155–163. https://doi.org/10.1016/j.soc.2017.08.006 (2018).
-
St John, E. R. et al. Diagnostic accuracy of intraoperative techniques for margin assessment in breast cancer surgery: A meta-analysis. Annals Surg. 265, 300–310. https://doi.org/10.1097/sla.0000000000001897 (2017).
-
Moher, D., Liberati, A., Tetzlaff, J., Altman, D. G. & Group, P. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Int. J. Surg. 8, 336–341. https://doi.org/10.1016/j.ijsu.2010.02.007 (2010).
-
Whiting, P. F. et al. QUADAS-2: A revised tool for the quality assessment of diagnostic accuracy studies. Ann. Intern. Med. 155, 529–536. https://doi.org/10.7326/0003-4819-155-8-201110180-00009 (2011).
-
Macaskill, P., Gatsonis, C., Deeks, J., Harbord, R. & Takwoingi, Y. Cochrane handbook for systematic reviews of diagnostic test accuracy. Version 1. 0., (London: The Cochrane Collaboration, 2010).
-
Higgins, J. P., Thompson, S. G., Deeks, J. J. & Altman, D. G. Measuring inconsistency in meta-analyses. BMJ Clin. Res. ed. 327, 557–560. https://doi.org/10.1136/bmj.327.7414.557 (2003).
-
Deeks, J. J., Macaskill, P. & Irwig, L. The performance of tests of publication bias and other sample size effects in systematic reviews of diagnostic test accuracy was assessed. J. Clin. Epidemiol. 58, 882–893. https://doi.org/10.1016/j.jclinepi.2005.01.016 (2005).
-
Yun, B. et al. Using a mobile device for margin assessment of specimen mammography in breast-conserving surgery. Medicine 100, e27243. https://doi.org/10.1097/md.0000000000027243 (2021).
-
Lin, C. et al. The application of intraoperative specimen mammography for margin status assessment in breast-conserving surgery: A single-center retrospective study. Breast J. 26, 1871–1873. https://doi.org/10.1111/tbj.13835 (2020).
-
Mariscotti, G. et al. Intraoperative breast specimen assessment in breast conserving surgery: Comparison between standard mammography imaging and a remote radiological system. Br. J. Radiol. 93, 20190785. https://doi.org/10.1259/bjr.20190785 (2020).
-
Park, K. U. et al. Digital breast tomosynthesis for intraoperative margin assessment during breast-conserving surgery. Ann. Surg. Oncol. 26, 1720–1728. https://doi.org/10.1245/s10434-019-07226-w (2019).
-
Pop, M. M., Cristian, S., Hanko-Bauer, O., Ghiga, D. V. & Georgescu, R. Obtaining adequate surgical margin status in breast-conservation therapy: intraoperative ultrasound-guided resection versus specimen mammography. Clujul Med 1957(91), 197–202. https://doi.org/10.15386/cjmed-891 (2018).
-
Naz, S. et al. Accuracy of specimen radiography in assessing complete local excision with breast-conservation surgery. Asian Pac. J. Cancer Prev. APJCP 19, 763–767. https://doi.org/10.22034/apjcp.2018.19.3.763 (2018).
-
Miller, C. L. et al. Comparison of intra-operative specimen mammography to standard specimen mammography for excision of non-palpable breast lesions: A randomized trial. Breast Cancer Res. Treat. 155, 513–519. https://doi.org/10.1007/s10549-016-3700-8 (2016).
-
Hisada, T. et al. Impact of intraoperative specimen mammography on margins in breast-conserving surgery. Mol. Clin. Oncol. 5, 269–272. https://doi.org/10.3892/mco.2016.948 (2016).
-
Chagpar, A. B. et al. Does three-dimensional intraoperative specimen imaging reduce the need for re-excision in breast cancer patients? A prospective cohort study. Am. J. Surg. 210, 886–890. https://doi.org/10.1016/j.amjsurg.2015.05.018 (2015).
-
Layfield, D. M. et al. The effect of introducing an in-theatre intra-operative specimen radiography (IOSR) system on the management of palpable breast cancer within a single unit. Breast 21, 459–463. https://doi.org/10.1016/j.breast.2011.10.010 (2012).
-
Bathla, L., Harris, A., Davey, M., Sharma, P. & Silva, E. High resolution intra-operative two-dimensional specimen mammography and its impact on second operation for re-excision of positive margins at final pathology after breast conservation surgery. Am. J. Surg. 202, 387–394. https://doi.org/10.1016/j.amjsurg.2010.09.031 (2011).
-
Weber, W. P. et al. Accuracy of frozen section analysis versus specimen radiography during breast-conserving surgery for nonpalpable lesions. World J. Surg. 32, 2599–2606. https://doi.org/10.1007/s00268-008-9757-8 (2008).
-
Goldfeder, S., Davis, D. & Cullinan, J. Breast specimen radiography: Can it predict margin status of excised breast carcinoma?. Acad. Radiol. 13, 1453–1459. https://doi.org/10.1016/j.acra.2006.08.017 (2006).
-
Coombs, N. J., Vassallo, P. P., Parker, A. J. & Yiangou, C. Radiological review of specimen radiographs after breast localisation biopsy is not always necessary. Eur. J. Surg. Oncol. 32, 516–519. https://doi.org/10.1016/j.ejso.2006.02.019 (2006).
-
Łuczyńska, E. The value of mammographic examination of the excised sample after open biopsy for the estimation of the surgical margins. Nowotwory 55, 303–309 (2005).
-
McCormick, J. T., Keleher, A. J., Tikhomirov, V. B., Budway, R. J. & Caushaj, P. F. Analysis of the use of specimen mammography in breast conservation therapy. Am. J. Surg. 188, 433–436. https://doi.org/10.1016/j.amjsurg.2004.06.030 (2004).
-
Gombos, E. V. et al. Two-view specimen radiography in assessing the surgical margins of breast carcinoma. J. Women’s Imaging 6, 16–22 (2004).
-
Saarela, A. O. et al. Wire-guided excision of non-palpable breast cancer: Determinants and correlations between radiologic and histologic margins and residual disease in re-excisions. Breast 10, 28–34. https://doi.org/10.1054/brst.2000.0174 (2001).
-
Graham, R. A. et al. The efficacy of specimen radiography in evaluating the surgical margins of impalpable breast carcinoma. AJR Am. J. Roentgenol. 162, 33–36. https://doi.org/10.2214/ajr.162.1.8273685 (1994).
-
Azu, M., Abrahamse, P., Katz, S. J., Jagsi, R. & Morrow, M. What is an adequate margin for breast-conserving surgery? Surgeon attitudes and correlates. Ann Surg Oncol 17, 558–563. https://doi.org/10.1245/s10434-009-0765-1 (2010).
-
Taghian, A. et al. Current perceptions regarding surgical margin status after breast-conserving therapy: Results of a survey. Ann. Surg. 241, 629–639. https://doi.org/10.1097/01.sla.0000157272.04803.1b (2005).
-
Li, W. H. & Li, X. R. Development of intraoperative assessment of margins in breast conserving surgery: A narrative review. Gland Surg. 11, 258–269. https://doi.org/10.21037/gs-21-652 (2022).
-
Esbona, K., Li, Z., Wilke, L. G. (2012) Intraoperative imprint cytology and frozen section pathology for margin. D-9420840, T—ppublish.
-
Ota, K., Rivera, C. & Martin, M. Specimen mammography distorts margin status in patients undergoing breast conserving surgery for early-stage breast cancer. Breast J. 23, 760–761. https://doi.org/10.1111/tbj.12920 (2017).
-
Moran, M. S. et al. Society of surgical oncology-American society for radiation oncology consensus guideline on margins for breast-conserving surgery with whole-breast irradiation in stages I and II invasive breast cancer. Ann. Surg. Oncol. 21, 704–716. https://doi.org/10.1245/s10434-014-3481-4 (2014).
-
Morrow, M. et al. Society of surgical oncology-american society for radiation oncology-american society of clinical oncology consensus guideline on margins for breast-conserving surgery with whole-breast irradiation in ductal carcinoma in situ. Ann. Surg. Oncol. 23, 3801–3810. https://doi.org/10.1245/s10434-016-5449-z (2016).
-
Bundred, J. R. et al. Margin status and survival outcomes after breast cancer conservation surgery: Prospectively registered systematic review and meta-analysis. BMJ Clin. Res. ed. 378, e070346. https://doi.org/10.1136/bmj-2022-070346 (2022).
-
Mendelson, E. B. Evaluation of the postoperative breast. Radiol. Clin. North Am. 30, 107–138 (1992).
-
Stomper, P. C., Davis, S. P., Weidner, N. & Meyer, J. E. Clinically occult, noncalcified breast cancer: Serial radiologic-pathologic correlation in 27 cases. Radiology 169, 621–626. https://doi.org/10.1148/radiology.169.3.2847231 (1988).
-
Jin, M. et al. Intraoperative specimen mammography for margin assessment in breast-conserving surgery. J Breast Cancer 22, 635–640. https://doi.org/10.4048/jbc.2019.22.e58 (2019).
-
Bau, M. G. et al. Vacuum intraoperative specimen mammography: A novel technique. Eur. J. Obstet. Gynecol. Reprod. Biol. 253, 1–6. https://doi.org/10.1016/j.ejogrb.2020.07.004 (2020).
-
Urano, M. et al. Digital mammography versus digital breast tomosynthesis for detection of breast cancer in the intraoperative specimen during breast-conserving surgery. Breast Cancer 23, 706–711. https://doi.org/10.1007/s12282-015-0628-5 (2016).
-
Janssen, N. N. Y. et al. Feasibility of micro-computed tomography imaging for direct assessment of surgical resection margins during breast-conserving surgery. J. Surg. Res. 241, 160–169. https://doi.org/10.1016/j.jss.2019.03.029 (2019).
Acknowledgements
This work was supported by the National Natural Science Foundation of China (Grant Numbers 81602716).
Author information
Authors and Affiliations
Contributions
The manuscript was drafted by L.C.. L.C. and P.T. developed the search strategies. L.C. and W.K.Y. independently screened eligible studies, extracted data from included studies. When there was disagreement between L.C. and W.K.Y., they discussed these articles with a senior author P.T.. QUADAS-2 checklist was independently completed by C.H.L. and X.Y.H.. All authors participated in the data synthesis. P.T. and C.Y.D. supervised all phases of this study. Authors all approved its final version.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Lin, C., Wang, Ky., Chen, Hl. et al. Specimen mammography for intraoperative margin assessment in breast conserving surgery: a meta-analysis. Sci Rep 12, 18440 (2022). https://ift.tt/QmiepMt
-
Received:
-
Accepted:
-
Published:
-
DOI: https://ift.tt/QmiepMt
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.
"breast" - Google News
November 02, 2022 at 05:26PM
https://ift.tt/nrqxtJZ
Specimen mammography for intraoperative margin assessment in breast conserving surgery: a meta-analysis | Scientific Reports - Nature.com
"breast" - Google News
https://ift.tt/E9bY4c5
https://ift.tt/Ndi0PMG
Bagikan Berita Ini














0 Response to "Specimen mammography for intraoperative margin assessment in breast conserving surgery: a meta-analysis | Scientific Reports - Nature.com"
Post a Comment